Hope is a precious thing in the medical industry.
“You go into your doctor and ask, ‘What are you going to do for me?’ and they say, ‘Nothing…’”
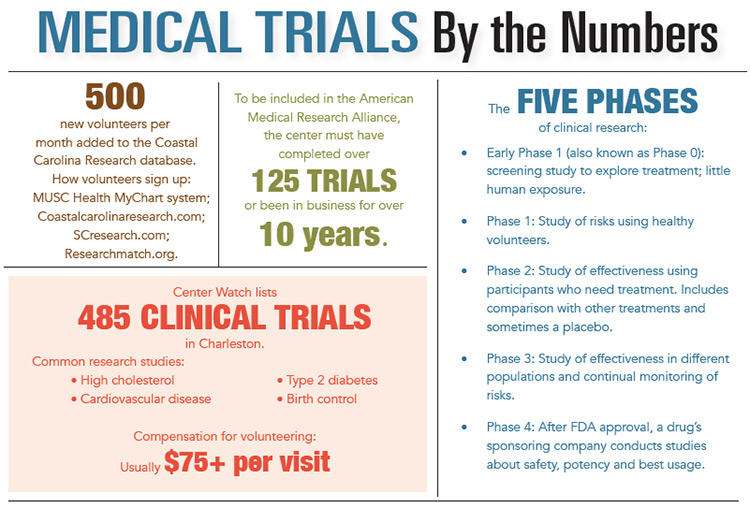
That is a bleak picture described by CEO Sean Rice of Medical Research South in Charleston. Unfortunately, that scene is repeated all too often. By some estimates, there are as many as 485 clinical trials underway in the Charleston area. They may not all be lifesaving or revolutionary or even end in the approval of a new treatment, but every single trial is working to deliver hope.

“I don’t want to be a guinea pig.”
That’s an unfortunate gut reaction, though not unheard of, according to clinical research professionals such as Rice. The second people hear “clinical trials,” they automatically write the topic off as irrelevant to their lives.
“In my opinion, it’s largely rooted in the lack of understanding about clinical research,” said Rice. “If you’ve ever been given a prescription, it’s been through clinical trials.”
A prescription’s journey from lab to pharmaceutical bottle takes at least five phases of research, and every phase involves clinical studies. Early phase 1 is purely for exploration. Through screenings and micro-doses, with very little human exposure, researchers explore the basic effects of a treatment before venturing into its potential or use. Next, later in phase 1, a treatment’s health effects are monitored for safety. Phases 2 and 3 examine effectiveness compared with other available treatments, side effects and how treatments affect different populations. Finally, after the FDA has approved a drug to be marketed, phase 4 studies gather information about a treatment’s overall safety, effectiveness and best application. The final phases are the most likely to involve thousands of volunteers at clinical trial centers, many of them in South Carolina and Charleston.

“We are looking for volunteers just like you,” says the website for Coastal Carolina Research. An average of 500 people join the database every month. “Both healthy volunteers and those with specific health conditions are needed to help answer important questions impacting the health of our friends and family,” says Outreach Coordinator, Jonathan Kiser.
Every time someone receives treatment, you can be sure the drug went through those five phases of studies and was vetted by “volunteers just like you.” Even seemingly ordinary treatments require trials.
“The impact of new drugs can be life-changing for millions of people, and we take great pride in that fact. Just one new drug for Type 2 diabetes, hot flashes or chronic lower back pain can make a huge difference in someone’s life, and that’s what clinical research is all about,” Kiser continues.
Coastal Carolina Research has worked on a number of “blockbuster drugs.” Some of the biggest-name prescriptions to come out of Charleston studies include Humera and Celebrex. Every trial comes with a specific goal and a pharmaceutical sponsor that meticulously selects research centers based on the resumes of their staff, the equipment in their centers, their past studies and the pool of volunteers who might qualify. Charleston is nearly always a strong contender, thanks not only to the Medical University of South Carolina but also to the large number of research facilities scattered across the region. “We’ve been fortunate to work on over 500 medical trials since 1997,” said Kiser, including vaccines for bubonic plague, anthrax, cholera and meningitis.
Research centers around Charleston and across the state are constantly running trials. Since 2004, South Carolina has been home to the Health Sciences South Carolina Group, the nation’s first-ever statewide collaborative for medical research. Health Sciences includes huge medical centers such as MUSC, Palmetto Health and Greenville Health, as well as Clemson University and the University of South Carolina. But most of the studies, even at these high-profile centers, fly under the radar.
Coastal Carolina Research does outreach through Facebook and other social media, will exhibit at 17 events in the lowcountry this year and just returned from the week-long World Vaccine Conference in Washington, D.C. Medical Research South, meanwhile, works with a wide network of local doctors to find volunteers who qualify. Still, the estimate of 485 clinical trials in Charleston probably surprises most residents. What are all these studies?
In Charleston today, you can find studies taking a look at chronic low back pain, Type 2 diabetes, rosacea, hypertension, birth control, high cholesterol and cardiovascular disease. Upcoming are more women’s issues like breast density, as well as studies on acne and frequent birth control trials. The list goes on like an encyclopedia of ailments and human conditions, some more treatable than others.
“We work with newborns through geriatric and everything in between,” said Rice of Medical Research South. “Today, when there is a huge underinsured or uninsured population, clinical trials can be a strong draw for a lot of people.”
Imagine health care that pays you. Qualifying participants – those who meet the sponsoring pharmaceutical company’s standards regarding age, conditions, other prescriptions and medical history – receive a full spectrum of compensation.
Medical research centers are often able to compensate volunteers for their time and travel.
Money makes clinical trials well worth the effort for many people. In a nation where health care costs and insurance are making the front page of newspapers nationwide every day, receiving treatment with no deductible is a huge draw. Volunteers receive all related health care free of charge, and that can include X-rays, blood work, EKGs and any relevant medication or doctor’s visits. There is no insurance company involved, zero payment to the clinical research company, and, in many cases, participants are compensated up to $100 per visit. The length and depth of trials varies, of course, with some requiring just a phone call to check in a year later and others needing in-depth journaling and hours of doctor’s visits.
“One of the benefits of trial research is the volunteer receives in-depth individual attention about their condition and care that they may not get in a typical visit to the primary care doctor,” said Kiser.
The monetary value of trials is undeniable. But health, in spite of insurance woes and health care bills, is still something that many people think of as priceless.
“Our tagline is: be a medical hero,” Kiser commented. “Our volunteers are everything to us. As such, you have an opportunity to help advance research and improve lives.”
What clinical trials really provide is hope. There’s no guarantee of a miracle cure – which Kiser stresses in every conversation. There are always risks, too.
“Volunteers must sign a legal document called informed consent,” Rice explained. “It lists negatives, including all the known side effects. But this is the same as those side effects printed in a tiny font on any prescription. Trials are the same. I remind volunteers of that so they’re comparing apples to apples.”
Every one of the hundreds of trials in Charleston alone shares the same core purpose – human treatment.
“You go into your doctor and ask, ‘What are you going to do for me?’ and they say, ‘Nothing.’”
It comes back to hope, said Rice.
“When your options are ‘nothing’ or ‘I’m going to advance medicine’… that’s what we do.”